The Soil: Soil Chemistry
The smallest soil particles determine the chemical characteristics of a soil.
The most important particles are the fine clay minerals (0.2 - 1 μm), which have a relatively large surface area per unit volume.
Kaolinite is a common clay mineral.
The surface area of clay particles may be up to 800 m/g (i.e. several orders of magnitude greater than for sands or silts).
They generally carry a negative surface charge and thus can bind both water and various important cations (e.g. NH4+, Ca2+, Mg2+, K+).
In addition to the mineral component of soil, organic matter, called humus, is also a source of chemical nutrients for plants.
Kaolinite is a common clay mineral composed of compound layers of ions (silicon, hydroxyl and aluminium ions). The different layers are held together by hydrogen bonding.
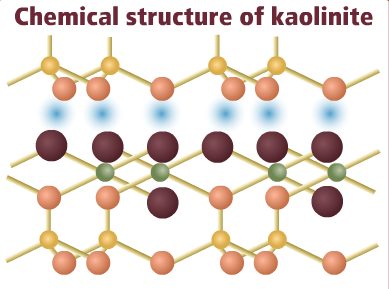
Select the different components of the Kaolinite mineral to view them in the diagram:
Silicon ion
Oxygen ion
Hydroxyl ion
Aluminium ion
Hydrogen bonding between layers
