Ecotypes: Fe Availability
It is known that PH affects the solubility (and therefore availability) of certain ions, including iron (Fe),
and that Fe deficiency commonly causes chlorosis in plants.
At high pH, Fe (as Fe3+) precipitates from solution as Fe(OH3)
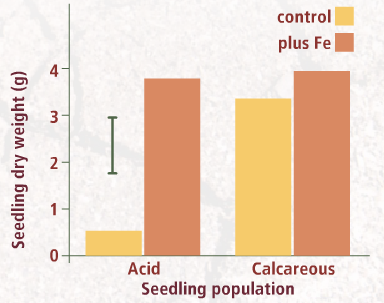
In a final experiment, Fe was added to pots containing the calcareous soil (pots containing untreated soil acted as the control).
The Fe was added in solution as chelate, which remains soluble even at high PH so that it remains available to roots even when added to the calcareous soil.
The result are shown in the accompanying graph.
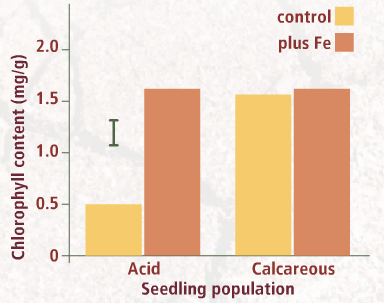
In addition to significantly improving plant growth, increasing Fe availability of seedlings from the acid population also prevented chlorosis. What effect is added Fe likely to have had on the chlorophyll content of these seedlings' leaves?
Move the mouse over the different elements in the graph for an explanation of the results.
View results of a total chlorophyll analysis
